Russia's COVID-19 Vaccine Released To The Public, Starts Trial In India This Month
Aadhya Khatri - Sep 10, 2020
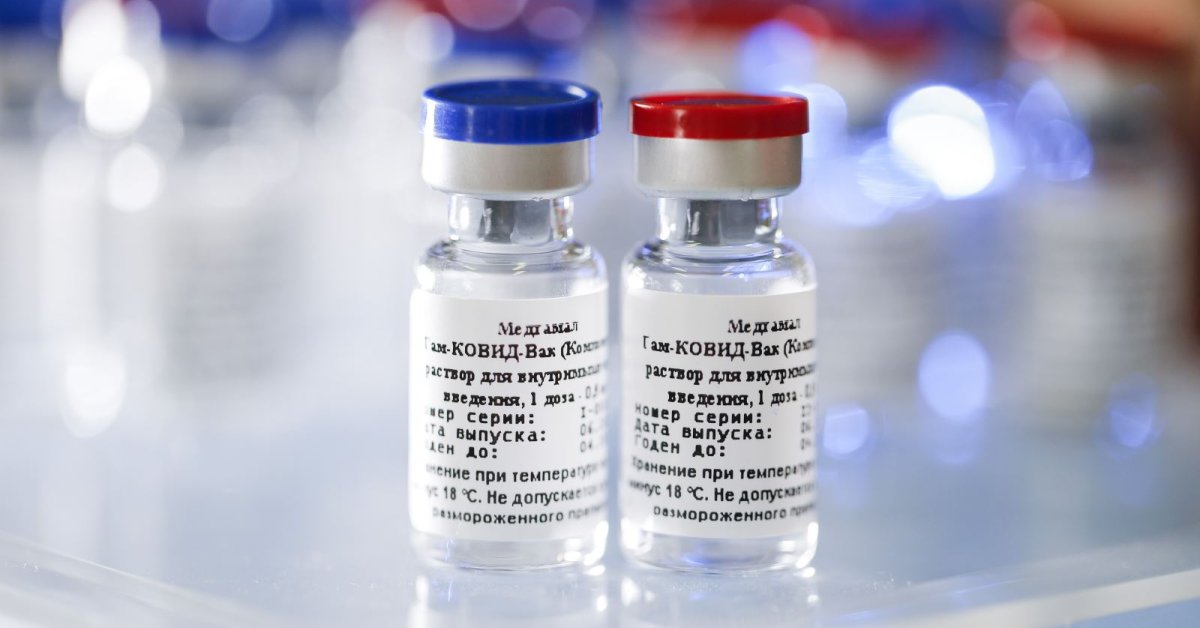
According to Russia Health Ministry, the first batch of the Sputnik V vaccine has been released into civil circulation after it passed all the needed tests
- This Man's Super-Antibody Can Be Diluted 10,000 Times But Still Works Against COVID-19
- These Indian Cities Are Under Lockdown Again In 2021
- India To Review Covishield Vaccine After Report Of Blood Clots Following Vaccination
COVID-19 is still wreaking havoc in India with the number of cases piling up day by day. When the second wave hits the country, our best hope now lies with a potential vaccine.
Two days ago, Russia made public that it had already started its vaccine trials.
According to Russia’s Health Ministry, the first batch of the Sputnik V has been released into civil circulation after it passed all the needed tests of Roszdravnadzor (or the Federal Service for Surveillance in Healthcare).
The Sputnik V vaccine was registered on August 11 and Putin told his citizens that it’s safe to use and effective against the novel COVID-19 pandemic.
Some other reports revealed that his own daughter was vaccinated with Sputnik V and she remains healthy after the shot.
Concerns Still Persist
Although Russian authorities assured the public that the vaccine is safe to use it does what it’s supposed to do, concerns still exist mostly because it hasn’t been adequately tested and it has been only a few months from when it was under development to its lunch.

Sputnik V’s sample size of initial phases was thought to be too small and at the moment, we haven’t had the results of the large phase 3 trial yet.
Russian Vaccine Trial In India
Some reports surfaced recently claimed that the Russian Sputnik V would go into trial in India this month.
On Monday, Russian Direct Investment Fund’s CEO Kirill Dmitriev said the post-registration studies with the participation of 40,000 people began on August 26, before the Phase 3 trial of AstraZeneca in the U.S which involved 30,000 people.
He also shared that clinical trials in the United Arab Emirates, Saudi Arabia, Brazil, the Philippines, and India would start this month.
Phase 3’s preliminary results will be out in October or November this year.
>>> Oxford's COVID-19 Vaccine Phase-3 Trials Paused Due To Serious Adverse Effect
Featured Stories

Features - Jan 29, 2026
Permanently Deleting Your Instagram Account: A Complete Step-by-Step Tutorial

Features - Jul 01, 2025
What Are The Fastest Passenger Vehicles Ever Created?

Features - Jun 25, 2025
Japan Hydrogen Breakthrough: Scientists Crack the Clean Energy Code with...

ICT News - Jun 25, 2025
AI Intimidation Tactics: CEOs Turn Flawed Technology Into Employee Fear Machine

Review - Jun 25, 2025
Windows 11 Problems: Is Microsoft's "Best" OS Actually Getting Worse?

Features - Jun 22, 2025
Telegram Founder Pavel Durov Plans to Split $14 Billion Fortune Among 106 Children

ICT News - Jun 22, 2025
Neuralink Telepathy Chip Enables Quadriplegic Rob Greiner to Control Games with...

Features - Jun 21, 2025
This Over $100 Bottle Has Nothing But Fresh Air Inside

Features - Jun 18, 2025
Best Mobile VPN Apps for Gaming 2025: Complete Guide

Features - Jun 18, 2025
A Math Formula Tells Us How Long Everything Will Live
Read more

Mobile- Feb 16, 2026
Xiaomi Launches Affordable Tracker to Compete with Apple's AirTag
For users tired of ecosystem lock-in or high prices, the Xiaomi Tag represents a compelling, no-frills option that delivers core functionality at a fraction of the cost.

ICT News- Feb 15, 2026
X Platform Poised to Introduce In-App Crypto and Stock Trading Soon
X has been laying the groundwork for this expansion.

Mobile- Feb 17, 2026
Anticipating the Samsung Galaxy S26 and S26+: Key Rumors and Specs
The Samsung Galaxy S26 series is on the horizon, sparking excitement among tech enthusiasts.
Comments
Sort by Newest | Popular