India To Review Covishield Vaccine After Report Of Blood Clots Following Vaccination
Aadhya Khatri - Mar 17, 2021
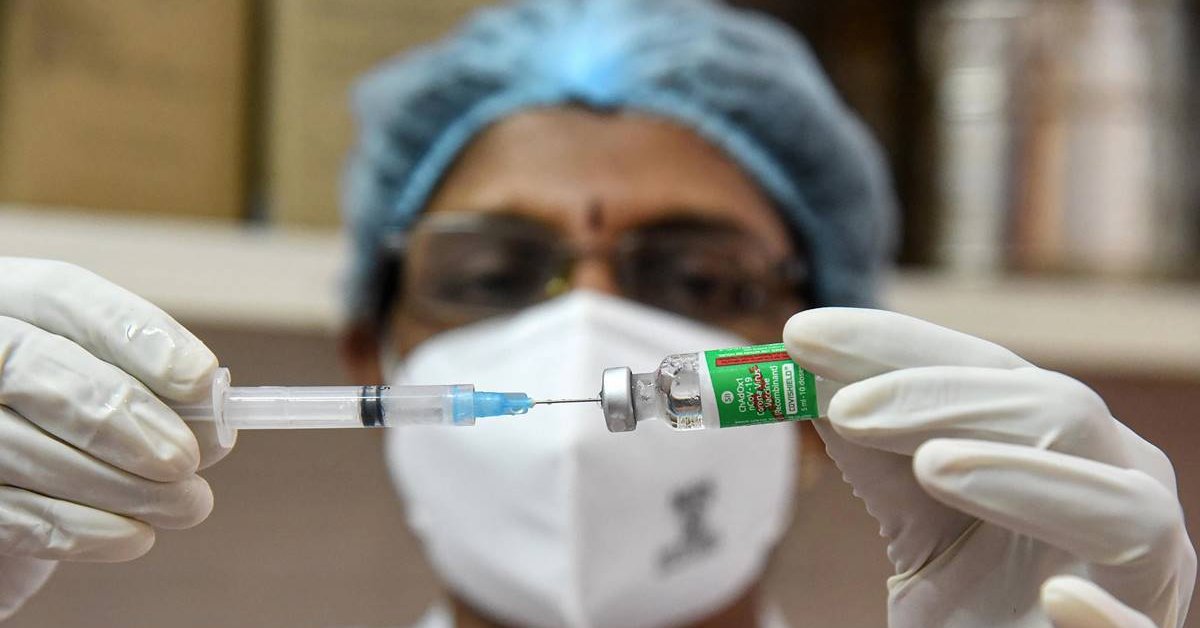
India’s drug regulators will reportedly review a vaccine candidate for the COVID-19 drive after news surfaced of blood illness happening to some recipients
- This Man's Super-Antibody Can Be Diluted 10,000 Times But Still Works Against COVID-19
- These Indian Cities Are Under Lockdown Again In 2021
- Researchers Developled Virtual Phone Virus To Recreate How COVID-19 Spreads
India’s drug regulators will reportedly review the two vaccine candidates for the nation-wide COVID-19 drive after news surfaced of blood illness happening to some recipients.
Covishield is based on the AZD1222 vaccine created by experts at the University of Oxford with AstraZeneca Plc having the license for it. In India, Serum Institute had made an agreement with both the Indian government and AstraZeneca to manufacture and supply the vaccine.
Last week, Iceland, Denmark, and Norway paused their AZD1222 rollouts after some recipients experienced blood clots and low blood platelet counts.
According to doctor Sigurd Hortemo of the Norwegian Medicines Agency, it has not yet to be determined if the three cases in Norway are caused by the vaccine.
AstraZeneca has not commented anything on the issue which came on top of the slew of other problems it already had on its hands.
News Reports
Here is what Dr. C.N. Manjunath of the Sri Jayadeva Institute of Cardiovascular Sciences remarked about the vaccine:
On March 14, 2021, 29.7 million doses of vaccine have been delivered, according to the Union health ministry website.
N.K Arora of the National COVID-19 Task Force said that they were looking into every adverse event and ensured that there were no concerns at the moment as the number of cases in India is very low relative to the country’s population.
There are other reports of recipients experiencing heart attacks after taking the Covishield vaccination. However, it is not to say that the vaccine is the cause of the reaction.
When a heart attack happens, part of the heart is deprived of blood, which leads to damage to muscles.
AEFI Reports
The information on adverse events following vaccination the Ministry of Health and Family Welfare offered is incomplete. The last piece of news on such an event was the death of 46 people and 51 others hospitalized.
IndiaSpend also revealed that not all of these people had received post mortem procedures and the government has yet to receive complete investigation reports.
Arora said so far, there had been up to 60 deaths after vaccination and all of them were labeled ‘coincidental’ by the government.
Vaccine Technology
To make the AZD1222 or Covishield, researchers delete the adenovirus genes without which the virus cannot replicate. They also gave the virus the genes that enable it to develop spikes similar to those of the Coronavirus.
When this adenovirus enters the body, it takes over the biological machinery of the cell to make the spike protein of the Coronavirus. Once the body’s immune system notices the spikes, it will attack and destroy the affected cells all while ‘remembering’ the spike protein.
The next time the body recognizes the spike protein, it will immediately respond and prevent infection from taking place.
After decades of working with adenovirus vector vaccines, experts around the world found out about a potential issue with the method.
If the human body has developed resistance to the adenovirus itself, the efficiency of the vaccine might be affected. According to Satyajit Rath of the Indian Institute of Science Education and Research, the debate has yet to be settled. However, both he and epidemiologist Jacob John said this hurdle could be overcome by a larger dosage.
The fact that Covishield is a double-dose vaccine means it has to be administered yearly to make sure that recipients maintain a certain level of resistance to the virus. The repetition also exposes the body to the adenovirus vector and encourages the immune response development of the recipients.
Another solution is to use a combination of different vaccines, including Sputnik V, AZD1222, and Johnson & Johnson’s. This method is believed to trigger different immune responses since these vaccines contain different types of vectors.
Russia’s Sputnik V vaccines use subtypes Ad26 and Ad5 adenovirus vectors, which are said to boost immune response without allowing the body to get used to the vectors.
Covaxin – an inactivated virus vaccine, as well as Moderna’s and Pfizer’s - mRNA vaccine, don’t pose this same threat and can easily be administered repeatedly.
>>> With The New Coronavirus Variants, You Must Wear At Least Two Masks
Featured Stories

Features - Jan 29, 2026
Permanently Deleting Your Instagram Account: A Complete Step-by-Step Tutorial

Features - Jul 01, 2025
What Are The Fastest Passenger Vehicles Ever Created?

Features - Jun 25, 2025
Japan Hydrogen Breakthrough: Scientists Crack the Clean Energy Code with...

ICT News - Jun 25, 2025
AI Intimidation Tactics: CEOs Turn Flawed Technology Into Employee Fear Machine

Review - Jun 25, 2025
Windows 11 Problems: Is Microsoft's "Best" OS Actually Getting Worse?

Features - Jun 22, 2025
Telegram Founder Pavel Durov Plans to Split $14 Billion Fortune Among 106 Children

ICT News - Jun 22, 2025
Neuralink Telepathy Chip Enables Quadriplegic Rob Greiner to Control Games with...

Features - Jun 21, 2025
This Over $100 Bottle Has Nothing But Fresh Air Inside

Features - Jun 18, 2025
Best Mobile VPN Apps for Gaming 2025: Complete Guide

Features - Jun 18, 2025
A Math Formula Tells Us How Long Everything Will Live
Read more

Mobile- Mar 06, 2026
Samsung Galaxy S26 Series Sets New Pre-Order Records: Ultra Model Captures 70 Percent of Sales
With such robust initial demand, the Galaxy S26 series is poised to outperform its predecessors in total sales, solidifying Samsung's dominance in the premium smartphone market.

Mobile- Mar 08, 2026
Transforming Android: New Desktop Mode Makes Phones PC-Capable
This update marks an exciting era for Android, empowering users to do more with their everyday devices.

Gadgets- Mar 08, 2026
Best Budget Keyboards of 2026
These budget keyboards prove that you don't need to spend hundreds for a quality typing experience in 2026.

Mobile- Mar 07, 2026
Xiaomi Unveils Cutting-Edge 17 Series Smartphones and Teases Vision GT Hypercar
Xiaomi's MWC 2026 presentation highlights its ambition to dominate not just smartphones but also connected ecosystems and electric vehicles.
Comments
Sort by Newest | Popular