What Happens When Mercury Meets Aluminum?
Dhir Acharya - Dec 20, 2018

Check out the video to see what strange thing arise from the reaction between Mercury and Aluminum.
Mercury, a supposedly destructive chemical element, can react with almost every metal to form alloys through a process called amalgamation. And a scientist did a small experiment to see what would happen when we let mercury react with aluminum.
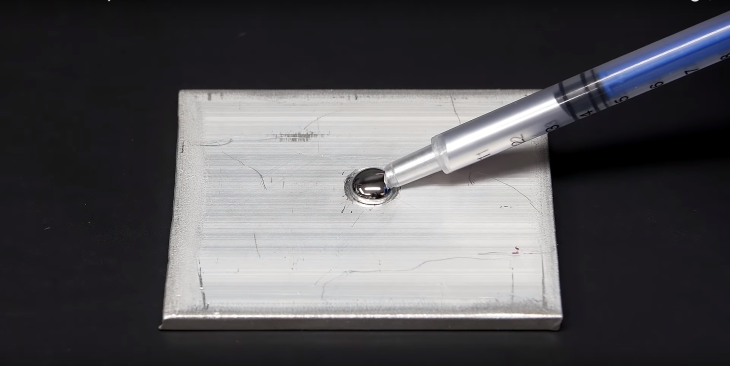
In preparation for the experiment, mostly, he got some mercury and purchased a strip of aluminum from Home Depot, which he then cut out a small rectangle piece and created a small crater in the middle. Mercury is known to be super annoying, that’s why there was a crater to stop it from rolling over and fall off the plate.
In normal conditions, there is a layer of oxide surrounding aluminum, which mercury cannot penetrate through. For some reaction to happen, the aluminum needs to actually touch the mercury, so the scientist tried several ways to get through this layer, like scratching and drilling through the oxide layer, but it was no use. He left the mercury on the aluminum plate for an hour, and there were no differences, so he decided to do it in a chemical way.
After taking all the mercury off the plate, he added some dilute hydrochloric acid to the crater, which reacted with the oxide layer and removed it. The acid was left there for a minute before it was wiped off.
Al2O3 + 6 HCl → 2 AlCl3 + 3 H2O
Al2O3: aluminum oxide
HCl: hydrochloric acid
AlCl3: Aluminum Chloride
H2O: Water
The scientist quickly added the mercury to the crater before the oxide layer could reform.
Reaction in dry condition
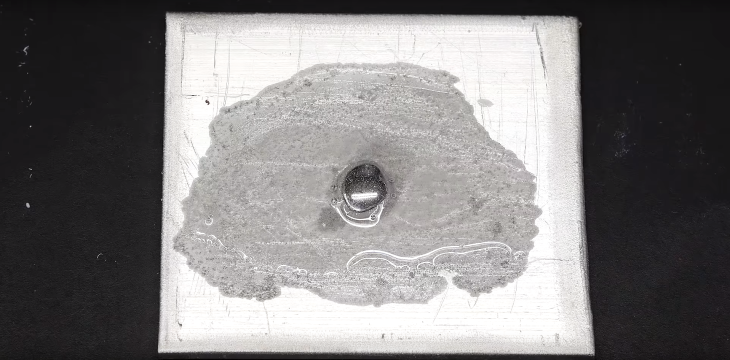
At this first attempt, almost as soon as the mercury was added, the amalgamation began, but we could barely see it. At a specially close look, it could be spotted that a few super small hairs were forming between the mercury and the other metal.
The amalgamation actually took really long in real-time, but here you can see the whole process in a time-lapse video, which is 180 times faster than reality. Accordingly, when the mercury met the aluminum, they combined and formed an amalgam, some of which got dissolved in the mercury and was brought to the top.
Hg + Al → Hg·Al
Al: Aluminum Metal
Hg: Mercury Metal
Hg.Al: Aluminum Amalgam
As it reached the top, the amalgam contacted and reacted with oxygen in the air, forming white aluminum oxide.
2 Al + 3 Hg2+ + 6 H2O → 2 Al(OH)3 + 6 H+ + 3 Hg
The amalgamation occurred for a short time only as the oxide layer formed quickly and protected it, preventing fibers from forming directly from the mercury. However, more fibers were growing from around because the mercury was partially dissolving into the aluminum. The amalgam growing from the outside also reacted with oxygen to form the oxide, but it does not shut itself down.
Theoretically, the amalgamation would keep going until the oxide covers the entire surface of the aluminum plate. Or the mercury could penetrate deeper into the aluminum and compromise its strength, which may take an extremely long time.
The result we got here, as you can see, is a tall white aluminum oxide “tower” consisting of fibers. Its structure, though, is really fragile and easy to collapse with a single poke. Plus, the whole thing weighs only 200 milligrams.
After the first time-lapse, the scientist cleaned up the surface of the plate and removed the mercury from the crater. Apparently, there was some fresh aluminum left around the oxide area, but that quickly reacted with oxygen again and turned grey. From that, we got the second time-lapse, there were fibers growing from the edges but not as much and still featured the weak structure. This time, when cleaning up the surface, there was no fresh aluminum left so we could say the amalgamation had ended. However, the formation of fibers may have stopped because the mercury amalgamated with aluminum is hidden under the oxide layer.
Reaction in water
In his second attempt, the scientist started things over. Once again, he dropped some dilute hydrochloric acid into the crater to remove the current oxide layer, then added the mercury back. The difference is that he added some water too.
The water prevented the formation of fiber. The amalgam did not react with oxygen anymore, and instead, it reacted with water to create Aluminum Hydroxide as well as Hydrogen Gas, as you can see in the following chemical equation.
2 Hg·Al + 6 H2O → 2 Al(OH)3 + 2 Hg + 3 H2
When the water was added, you could spot Hydrogen gas bubbles appearing from mercury. What to note, though, is that the Hydrogen bubbles came out from all over the water-contacted area, which means there was still some amalgam hidden under the grey layer.
However, this time, the mercury could not be cleaned up completely, there was some left on the plate. So the scientist decided to do another time-lapse. See the result.
The impact of the amalgamation on the aluminum plate
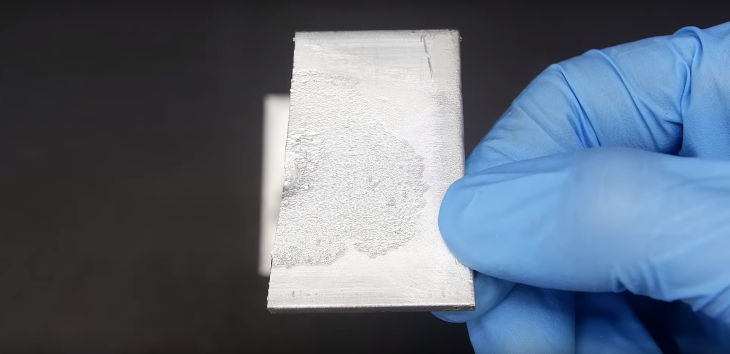
After wiping off everything, the scientist eventually cut the aluminum plate in half. From the side, it looks like the amalgamation only affected the plate on the surface level. No further damage was found when he cleaned its surface.
So, it’s likely that it would take a really long time for the mercury to actually damage the aluminum surface, or we would have to keep wiping off the oxide layer.
Application
We usually use aluminum amalgams for reduction reactions in chemistry. Under normal condition, metallic mercury failed to attack this metal, so for these reactions to occur, it’s necessary that we use soluble mercury salt.
What we can see here, especially in the reaction in the dry condition, the mercury keeps exposing fresh aluminum that donates electrons. One example of this reaction’s common application, unfortunately, an illegal one, is MDMA (MethyleneDioxyl-MethamphetAmine) production. For those who don’t know what MDMA is, it’s a type of heroin.
Now, you can watch the full video of this experiment here.
Featured Stories

Features - Jul 01, 2025
What Are The Fastest Passenger Vehicles Ever Created?

Features - Jun 25, 2025
Japan Hydrogen Breakthrough: Scientists Crack the Clean Energy Code with...

ICT News - Jun 25, 2025
AI Intimidation Tactics: CEOs Turn Flawed Technology Into Employee Fear Machine

Review - Jun 25, 2025
Windows 11 Problems: Is Microsoft's "Best" OS Actually Getting Worse?

Features - Jun 22, 2025
Telegram Founder Pavel Durov Plans to Split $14 Billion Fortune Among 106 Children

ICT News - Jun 22, 2025
Neuralink Telepathy Chip Enables Quadriplegic Rob Greiner to Control Games with...

Features - Jun 21, 2025
This Over $100 Bottle Has Nothing But Fresh Air Inside

Features - Jun 18, 2025
Best Mobile VPN Apps for Gaming 2025: Complete Guide

Features - Jun 18, 2025
A Math Formula Tells Us How Long Everything Will Live

Features - Jun 16, 2025





Comments
Sort by Newest | Popular