Initial Gene-Editing Trials Show Positive Results In Treating Blood Diseases
Dhir Acharya - Nov 22, 2019
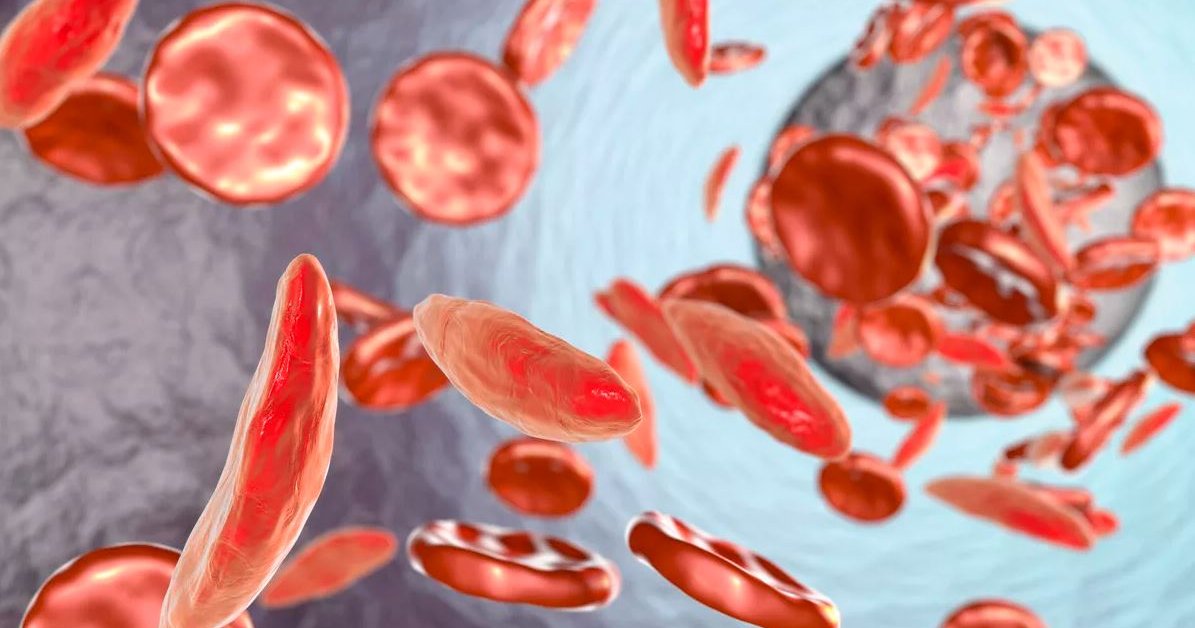
CRISPR Therapeutics and Vertex developed an investigational therapy called CTX001 that uses gene-editing tech CRISPR to edit stem cells in the human body.
- Scientists Rename Human Genes Because Excel Keeps Mistaking Them For Dates
- New Method Allows Scientists To Edit Genes From Inside Patient Body
- Chinese Scientist Who Created Gene-Edited Babies Gets A Three-Year Sentence
CRISPR Therapeutics along with Vertex has developed an investigational therapy called CTX001 that uses gene-editing tech CRISPR to edit stem cells in the human body. The treatment has given promising initial results to two patients with inherited blood diseases. Preliminary results suggest that this therapy is safe and sets the foundation for further trials of this new treatment.
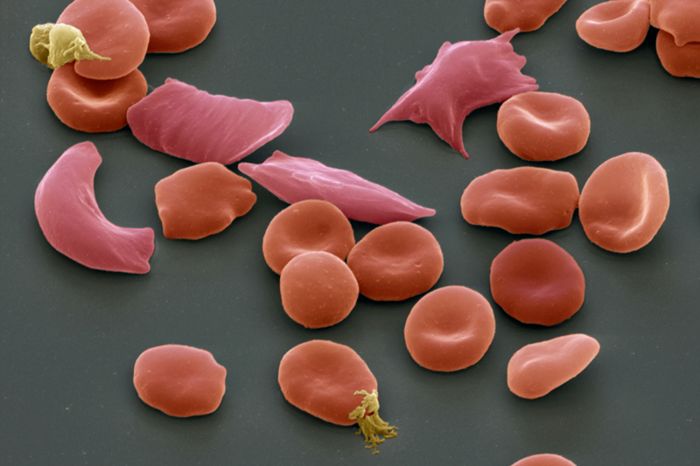
The patients in question have different hemoglobinopathies, the disorders affecting a protein called hemoglobin that carries O2 around the body. The first patient is from the US, having sickle cell disease that generates misshapen red blood cells that are sickle-shaped. The patient started receiving the treatment this April. The second patient is from Europe, who has beta-thalassemia, which reduces the amount of hemoglobin generated. This patient has been treated for nine months.
Patients with sickle cell disease suffer from painful crises when the sickle-shaped red blood cells block blood vessels. Meanwhile, patients who have beta-thalassemia need constant blood transfusions so that the hemoglobin levels in their bodies are properly managed.
After receiving the treatment, the first patient no longer had painful crises while the second one no longer required blood transfusions.
In a Tuesday press release, CRISPR Therapeutics CEO Samarth Kulkarni said that:

The treatments are not cheap or easy to carry out. They need health care professionals for collecting stem cells from patients’ blood before editing them using CRISPR, outside the body. In this gene-editing technology, a version of the hemoglobin gene is activated, which is normally deactivated in adults. In addition, the patients receive the edited gene through a stem cell transplant. Once the patient has received the cells, they are expected to create shop in the bone marrow then start to pump out adequate amounts of hemoglobin.
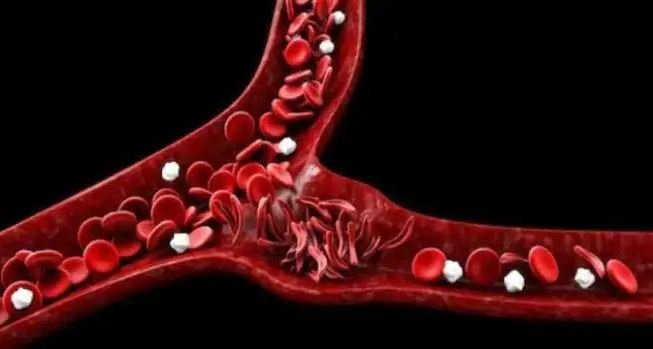
Vertex and CRISPR Therapeutics expect to enroll 45 patients from Europe, Canada, and the US in CTX001 trials, each of whom will receive long-term follow-up study for the treatment’s safety assessment.
Over the past year, CRISPR has been talked about a lot as scientists take ethical and moral concerns into account with the ability to edit the human gene. Last November, scientist He Jiankui from China revealed that he had used CRISPR to edit human embryos, which generated a gene-edited pair of twins. The scientific community slammed the conduct, calling to stop human gene editing, which has gathered steam gradually throughout this year.
However, this technology has also been known as a way to bring back extinct species or terminate an entire species of mosquitoes that carries malaria. And while ethicists and scientists are struggling with considerations, researchers are working to create more impressive, innovative techs to manipulate DNA with high accuracy. The CTX001 may unlock a new era for gene therapy.
Featured Stories

Features - Jan 29, 2026
Permanently Deleting Your Instagram Account: A Complete Step-by-Step Tutorial

Features - Jul 01, 2025
What Are The Fastest Passenger Vehicles Ever Created?

Features - Jun 25, 2025
Japan Hydrogen Breakthrough: Scientists Crack the Clean Energy Code with...

ICT News - Jun 25, 2025
AI Intimidation Tactics: CEOs Turn Flawed Technology Into Employee Fear Machine

Review - Jun 25, 2025
Windows 11 Problems: Is Microsoft's "Best" OS Actually Getting Worse?

Features - Jun 22, 2025
Telegram Founder Pavel Durov Plans to Split $14 Billion Fortune Among 106 Children

ICT News - Jun 22, 2025
Neuralink Telepathy Chip Enables Quadriplegic Rob Greiner to Control Games with...

Features - Jun 21, 2025
This Over $100 Bottle Has Nothing But Fresh Air Inside

Features - Jun 18, 2025
Best Mobile VPN Apps for Gaming 2025: Complete Guide

Features - Jun 18, 2025
A Math Formula Tells Us How Long Everything Will Live
Read more

Mobile- Feb 17, 2026
Anticipating the Samsung Galaxy S26 and S26+: Key Rumors and Specs
The Samsung Galaxy S26 series is on the horizon, sparking excitement among tech enthusiasts.

ICT News- Feb 18, 2026
Google's Project Toscana: Elevating Pixel Face Unlock to Rival Apple's Face ID
As the smartphone landscape evolves, Google's push toward superior face unlock technology underscores its ambition to close the gap with Apple in user security and convenience.

ICT News- Feb 19, 2026
Escalating Costs for NVIDIA RTX 50 Series GPUs: RTX 5090 Tops $5,000, RTX 5060 Ti Closes in on RTX 5070 Pricing
As the RTX 50 series continues to push boundaries in gaming and AI, these price trends raise questions about accessibility for average gamers.
Comments
Sort by Newest | Popular