Bill Gates Believes India Can Provide COVID-19 Vaccines For The Whole World
Harin - Jul 20, 2020

Bill Gates is confident that Indian pharmaceutical companies can produce enough COVID-19 vaccines for the entire world, not just India.
- When Closed Source Met Open Source: Bill Gates Finally Meets Linus Torvalds After 50 Years
- This Man's Super-Antibody Can Be Diluted 10,000 Times But Still Works Against COVID-19
- These Indian Cities Are Under Lockdown Again In 2021
The Indian pharmaceutical industry is racing to produce COVID-19 vaccines. And it has earned support from Bill Gates, the Microsoft co-founder, and philanthropist.
Bill Gates is confident that Indian pharmaceutical companies can produce enough COVID-19 vaccines for the entire world.

Gates thought highly of the capacity of the pharma industry of the country. For a long time, India has become the world’s vaccine manufacturing hub. The country has manufactured more vaccines than the rest of the world.
India has done a lot of extremely important things. Its pharma industry is working on the coronavirus vaccine using other great capacities used for other diseases. Speaking in the COVID-19: India’s War Against The Virus, Gates added that India had also been facing a huge health crisis because of the country’s size and population density.
He also praised the Serum Institute for its role as the world’s largest vaccine supplier and Bharat Biotech and Bio E for their COVID-19 vaccine developing efforts.

Serum India will be in charge of manufacturing the COVID-19 vaccine currently on human trials at Oxford University, the UK. The company’s goal is to produce four to five million doses of vaccines each month in the first six months. After that, they might scale up the production to 10 million doses monthly. By September-October, Serum Institute is planning to produce 20 to 40 million doses. By doing this, the product will be available in other countries as well.
Meanwhile, COVAXIN, the first indigenous COVID-19 vaccine of India, developed by Bharat Biotech has received approval to carry out human clinical trials.
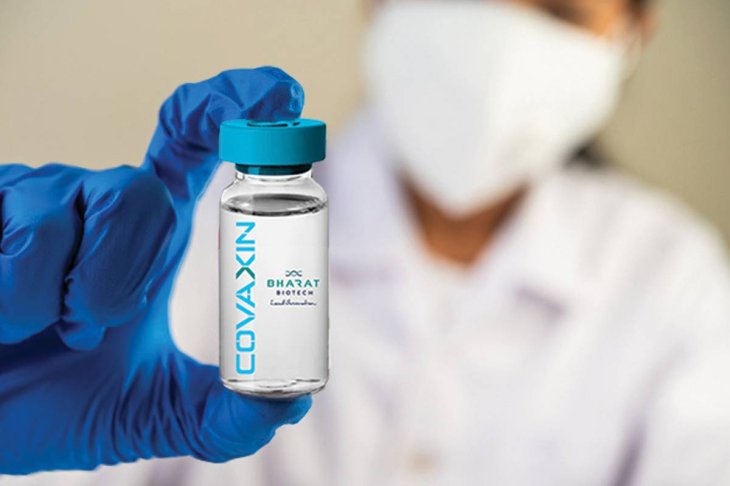
The Drug Controller General of India has allowed Bharat Biotech to conduct Phase I and II of human trials. As per the vaccine maker, its clinical trials of the vaccine will begin in July 2020. The company said that the vaccine’s development was in collaboration with the National Institute of Virology and Indian Council of Medical Research.
>>> Second Indian COVID-19 Vaccine Approved For Human Trials
Featured Stories

Features - Jan 29, 2026
Permanently Deleting Your Instagram Account: A Complete Step-by-Step Tutorial

Features - Jul 01, 2025
What Are The Fastest Passenger Vehicles Ever Created?

Features - Jun 25, 2025
Japan Hydrogen Breakthrough: Scientists Crack the Clean Energy Code with...

ICT News - Jun 25, 2025
AI Intimidation Tactics: CEOs Turn Flawed Technology Into Employee Fear Machine

Review - Jun 25, 2025
Windows 11 Problems: Is Microsoft's "Best" OS Actually Getting Worse?

Features - Jun 22, 2025
Telegram Founder Pavel Durov Plans to Split $14 Billion Fortune Among 106 Children

ICT News - Jun 22, 2025
Neuralink Telepathy Chip Enables Quadriplegic Rob Greiner to Control Games with...

Features - Jun 21, 2025
This Over $100 Bottle Has Nothing But Fresh Air Inside

Features - Jun 18, 2025
Best Mobile VPN Apps for Gaming 2025: Complete Guide

Features - Jun 18, 2025
A Math Formula Tells Us How Long Everything Will Live
Read more

Mobile- Feb 16, 2026
Xiaomi Launches Affordable Tracker to Compete with Apple's AirTag
For users tired of ecosystem lock-in or high prices, the Xiaomi Tag represents a compelling, no-frills option that delivers core functionality at a fraction of the cost.

ICT News- Feb 15, 2026
X Platform Poised to Introduce In-App Crypto and Stock Trading Soon
X has been laying the groundwork for this expansion.

Mobile- Feb 14, 2026
Android 17 Beta 1 Now Available for Pixel Devices
While Android 17 Beta 1 doesn't introduce flashy consumer-facing changes yet, it lays the groundwork for a more robust and flexible platform.
Comments
Sort by Newest | Popular