This Artificial Leaf Can Produce Gas From Only Carbon Dioxide, Water, And Sunlight
Aadhya Khatri - Nov 06, 2019
This artificial leaf is vital in closing the global cycle of carbon as well as setting up a more sustainable fuel industry
- These Artificial Leaves Can Absorb Sunlight To Produce Cheaper Medicine
- New Artificial Leaf Design Could Convert CO2 10 Times Better Than Real Ones
Recently, experts at the University of Cambridge came up with a way to produce a commonly used gas, which is currently made from fossil fuels, from only water, sunlight, and carbon dioxide.
The device to produce the gas, called syngas, is like an artificial leaf, and it works fine on overcast or cloudy days. The best part is, unlike the existing ways to make syngas, the artificial leaf releases no additional carbon dioxide to the environment.
Before this device is fabricated, syngas is produced from carbon monoxide and hydrogen. It plays an important part in making fuels, plastics, fertilizers, and pharmaceuticals.
According to Erwin Reisner, professor at Cambridge's Department of Chemistry, who has been working on this project for seven years, this artificial leaf is vital in closing the global cycle of carbon as well as setting up a more sustainable fuel industry. The project took inspiration from photosynthesis, the process that turns carbon dioxide into food with the energy from sunlight.
The leaf features two light absorbers and a catalyst made from cobalt, an element that can be found in a large amount in nature.
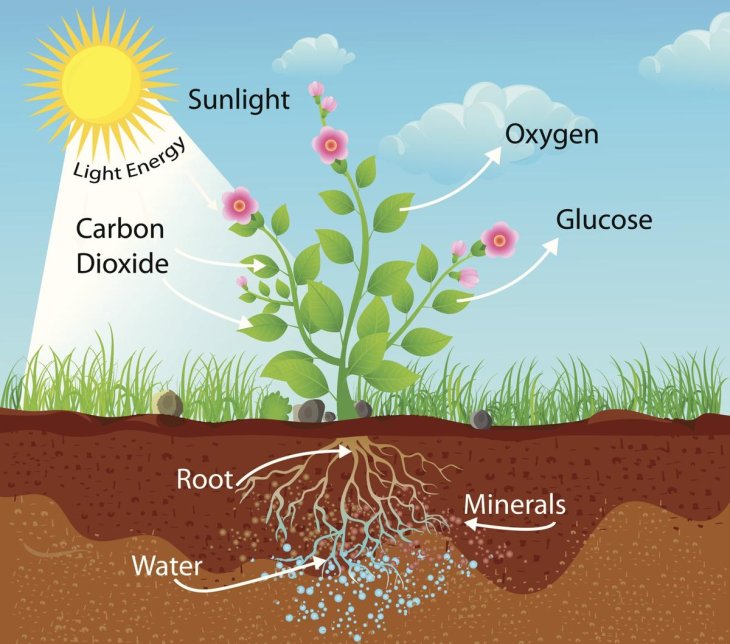
When the leaf is submerged in water, one of the two light absorbers produces oxygen by using the catalyst, while the other one makes hydrogen and carbon monoxide from water and carbon dioxide, the elements needed for syngas.
The experts also found out that the light absorbers are able to work in low light conditions on overcast days. According to Virgil Andrei, the paper’s first author, this means the artificial leaf is not limited to countries with abundant sunlight or summer months. It can be used anywhere, anytime.
The research is funded by OMV, an Austria-based petrochemical company, which is in search of a way to be more sustainable, as well as the Austrian government.
This is not the first artificial leaf ever created, but unlike that of experts from Cambridge University, they can only make hydrogen. What sets this leaf apart is its combination of materials and the catalyst.
The perovskite light absorbers can provide high electrical current and photovoltage to facilitate the transformation of carbon dioxide to carbon monoxide. The cobalt catalyst is also more efficient in producing carbon monoxide than that made from silver or platinum; plus, cobalt is cheaper and more abundant in nature.
What the team aims at now is a sustainable way to produce petrol. Reisner said that their ambition is to create fuel from water and carbon dioxide after just one step; instead of converting syngas into petrol.
We have had certain advancements in producing electricity from sustainable sources like sunlight or wind power, but currently, the production can meet around 25% of the demand for power. This calls for synthetic power. So according to Reisner, the artificial leaf is highly relevant, even when we have ways to lessen our reliance on fossil fuels.
Andrei said that they were trying to make ethanol, which can be used as fuel right away. It was hard to produce that substance after just one step from water and carbon dioxide but the team was confident that they were going in the right direction. They have had the catalyst right, and they believe that they will have the process ready in the near future.
Other organizations funding the project are the Engineering and Physical Sciences Research Council, the Biotechnology and Biological Sciences Research Council, and the Winton Programme for the Physics of Sustainability.
Featured Stories

Features - Jul 01, 2025
What Are The Fastest Passenger Vehicles Ever Created?

Features - Jun 25, 2025
Japan Hydrogen Breakthrough: Scientists Crack the Clean Energy Code with...

ICT News - Jun 25, 2025
AI Intimidation Tactics: CEOs Turn Flawed Technology Into Employee Fear Machine

Review - Jun 25, 2025
Windows 11 Problems: Is Microsoft's "Best" OS Actually Getting Worse?

Features - Jun 22, 2025
Telegram Founder Pavel Durov Plans to Split $14 Billion Fortune Among 106 Children

ICT News - Jun 22, 2025
Neuralink Telepathy Chip Enables Quadriplegic Rob Greiner to Control Games with...

Features - Jun 21, 2025
This Over $100 Bottle Has Nothing But Fresh Air Inside

Features - Jun 18, 2025
Best Mobile VPN Apps for Gaming 2025: Complete Guide

Features - Jun 18, 2025
A Math Formula Tells Us How Long Everything Will Live

Features - Jun 16, 2025
Comments
Sort by Newest | Popular