Scientists Convert CO2 Into Solid Coal At Room Temperature
Anita - Apr 30, 2019

A new technique of turning carbon dioxide into solid coal could revolutionise carbon capture and storage.
- Researchers Make Lighter & Biodegradable Drones With Pineapple Leaves
- Going Vegetarian Could Eliminate 16 Years Of CO2 Emissions By 2050
- This Artistic Solution Is Perfect To Reduce Air Pollution In India
Scientists have harnessed metals in liquid form to convert carbon dioxide (CO2) into the solid coal by a technique that could make a breakthrough in capturing and storing carbon as well as offer a new pathway for removing all greenhouse gas from our atmosphere.
The research team from Australia-based RMIT University created a liquid-metal electrocatalyst which, at room temperature, transforms gaseous CO2 into carbon-containing solids.

And the catalyst also prevents coking in which the surface of the catalyst is stuck with solid carbon, an issue in the previous research in this field.
The answer lies in the phrase “room temperature”. Preparing carbon nanomaterials with other methods requires temperatures of up to hundreds of degrees C, meaning they are energy-intensive and not feasible for commercial use.
Similarly, compressing carbon dioxide into a liquid then injecting it underground is costly and harmful to the environment with leaking risks from storage sites, according to the researchers.
In addition, cutting CO2 to products with high values like fuel, and chemical feedstocks does not keep the carbon permanently. For example, the fuels release CO2 again once they're burnt.
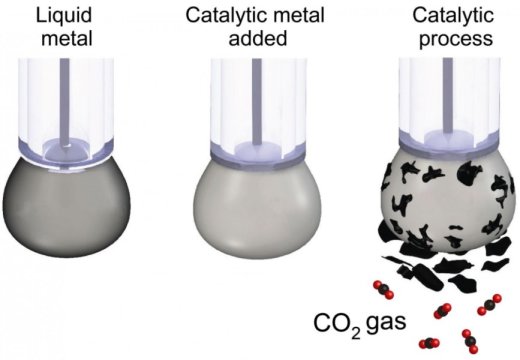
Using liquid metal to convert CO2 into solid coal
Torben Daeneke, an RMIT researcher, said that turning the gas form into the solid form is probably a more sustainable way. He said:

Dorna Esrafilzadeh, an RMIT colleague of Daeneke, developed an electrochemical method to capture and turn atmospheric CO2 into solid carbon which can be stored.
Esrafilzadeh and colleagues designed a liquid metal catalyst whose surface properties increase its efficiency in conducting electricity and still chemically activate the surface.
The team dissolved carbon dioxide in a beaker, which they filled with an electrolyte liquid, along with a little liquid metal later charged with an electrical current.
And the carbon dioxide slowly changes to solid carbon flakes naturally separated from the surface of the liquid metal, which allows the continuous carbonaceous solid production.
Esrafilzdeh said that the produced carbon can also act as an electrode. She said:

Daeneke stated that there is still more work to be accomplished, but the early findings can be seen as “a crucial first step to delivering solid storage of carbon.”
The cooperation included many scientists from China, Australia, the U.S., and Germany. The research findings are now published in the Nature Communications journal.
Featured Stories

Features - Jan 29, 2026
Permanently Deleting Your Instagram Account: A Complete Step-by-Step Tutorial

Features - Jul 01, 2025
What Are The Fastest Passenger Vehicles Ever Created?

Features - Jun 25, 2025
Japan Hydrogen Breakthrough: Scientists Crack the Clean Energy Code with...

ICT News - Jun 25, 2025
AI Intimidation Tactics: CEOs Turn Flawed Technology Into Employee Fear Machine

Review - Jun 25, 2025
Windows 11 Problems: Is Microsoft's "Best" OS Actually Getting Worse?

Features - Jun 22, 2025
Telegram Founder Pavel Durov Plans to Split $14 Billion Fortune Among 106 Children

ICT News - Jun 22, 2025
Neuralink Telepathy Chip Enables Quadriplegic Rob Greiner to Control Games with...

Features - Jun 21, 2025
This Over $100 Bottle Has Nothing But Fresh Air Inside

Features - Jun 18, 2025
Best Mobile VPN Apps for Gaming 2025: Complete Guide

Features - Jun 18, 2025
A Math Formula Tells Us How Long Everything Will Live
Read more

ICT News- Feb 22, 2026
Which AI Model Excels at Which Task in 2026: A Comprehensive Guide
In 2026, the best AI depends on your needs: Gemini for multimodal and speed, Claude for coding and reasoning, GPT for creativity, and Grok for straightforward tech insights.

ICT News- Feb 21, 2026
AI Coding Agent Causes Major AWS Outage at Amazon
In a striking example of the risks associated with deploying advanced AI in critical systems, Amazon Web Services (AWS) recently faced multiple outages attributed to its own AI coding assistants.

ICT News- Feb 23, 2026
It's Over for Xbox: Asha Sharma Takes Over to Ruin Microsoft Gaming with AI
It's not just a leadership change; it's the death knell for Xbox.
Comments
Sort by Newest | Popular